Share Your Story About the Organ Transplant System
We want to hear from doctors, nurses, technicians, patients and others with experience in the system. Tell us your experiences below.
THURSDAY, 18 APRIL 2024, 03:06
We want to hear from doctors, nurses, technicians, patients and others with experience in the system. Tell us your experiences below.
A Houston hospital is investigating whether a doctor altered a transplant list to make his patients ineligible for care. A disproportionate number of them have died while waiting for new organs.
We want to hear from pet owners about their experiences taking their animals to the vet, and how they paid for their animals’ care.
He and his wife wrote pioneering studies; he used the term “coercion control” to describe psychological and physical dominance by abusers.

Neurosurgeon Mansoor Foroughi alleges he was dismissed after he raised safety concerns.

The latest figures show many patients face long waits, but the PM insists headway is being made.
A public research institute in Brazil has proved a new shot protects against the disease, but can’t make it fast enough to stop the huge outbreak sweeping Latin America.
One company is going to great lengths to build it up, but it will be years before it returns to the minimum level.

Victoria Atkins is challenged to name some health trusts meeting their targets to cut waiting lists.
Young people are struggling with social comparison and self-criticism, but experts say there are ways to quiet those voices.
The campsite, run by a 22-year-old volunteer, became a first stop for people seeking food, water and warmth as they waited to be apprehended by border authorities.
The state’s Supreme Court ruled that the 1864 law is enforceable today. Here is what led to its enactment.

Scientists say the pilot scheme aims to make sure donated kidneys are less likely to be rejected.

Doctors at the Royal Stoke Hospital helped a 13-year-old girl with her rare liver condition.

Cannabis vapes are being sold containing the dangerous animal tranquiliser xylazine, experts say.
Five European countries have recently restricted hormone treatments for adolescents with gender distress. They have not banned the care, unlike many U.S. states.
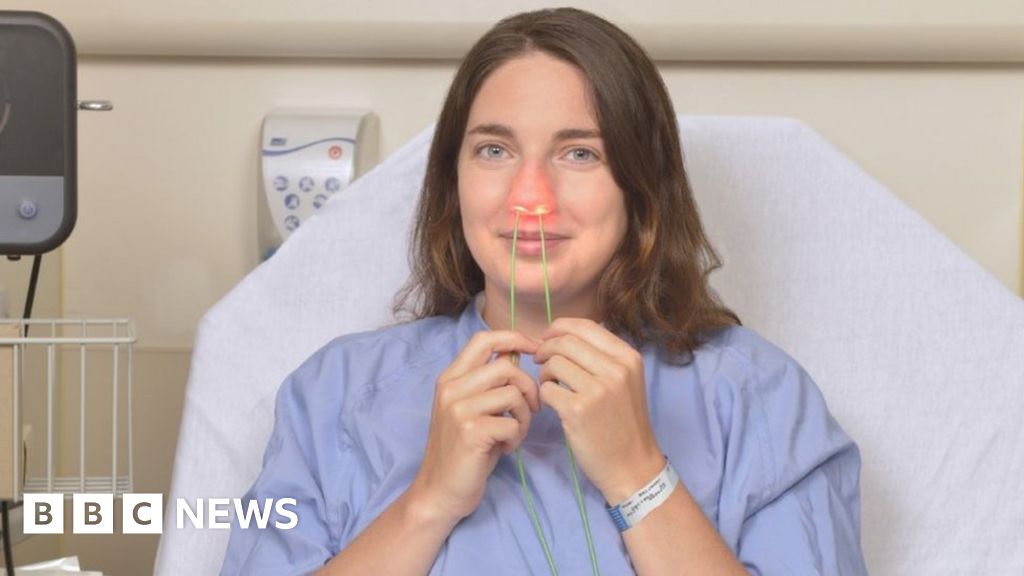
The light-activated antimicrobial quickly kills bugs including viruses, bacteria, and fungi.
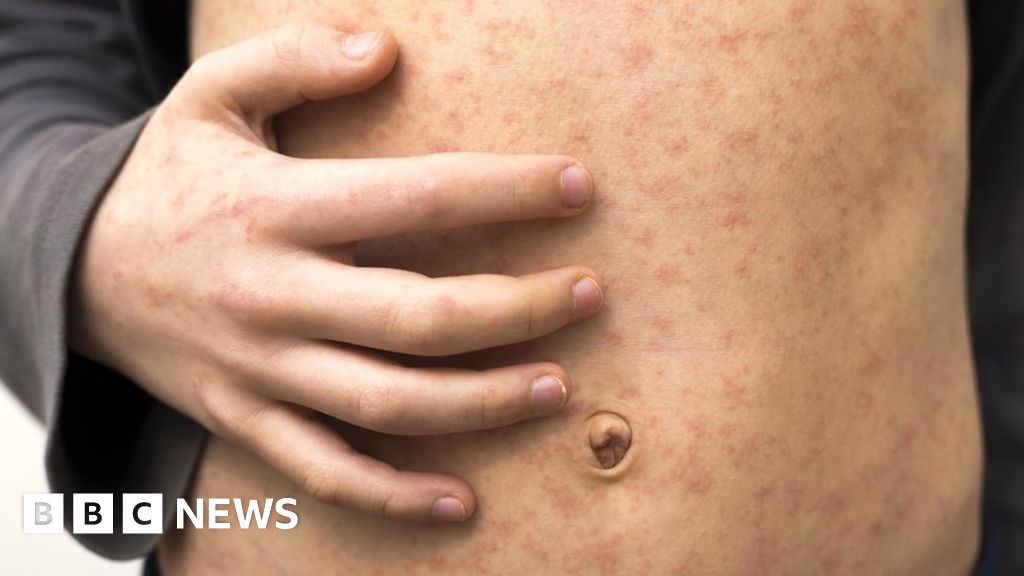
Four children in south-east Wales have the virus and are being treated, authorities say.
A citizen-science collaboration in New York has turned up a half-dozen birds infected with the avian flu virus.
Ireland will require them starting in 2026, and there are nascent efforts elsewhere to add more explicit labeling about the health risks of drinking.

The UK’s largest study of Covid patients treated in hospital found signs of active inflammation.